Prior to our 4th Honors Biology class, we were assigned to do a two things for homework.
First, we were asked to find out why red cabbage is a suitable pH indicator, how the chemistry of acids and bases affect this indicator, and explain how acid rain would affect red cabbage growth and development. The answer to the first part of this is that red cabbage contains a pigment molecule called flavin, which is an anthocyanin (an anthocyanin is known to turn red, purple, or blue depending on the pH). The answer to the second part of the question is that the colors of the pigments change color in response to changes in hydrogen ion concentration, or pH. When acids donate hydrogen ions to the solution, the hydrogen ion concentration of the solution increases. High hydrogen ion concentrations cause the pigments in the red cabbage indicator to turn red. When bases are added, the hydrogen ion concentration decreases, because the base accepts the hydrogen ions. Low hydrogen ion concentration causes the red cabbage indicator to turn greenish. A neutral hydrogen ion concentration would result in a purplish color. Finally, the answer to the last part of the question is that the red cabbage would grow and develop more slowly in acid rain, because acid rain contains less water than regular rain, making it harder to photosynthesis. Additionally, the acids would seep into the plant and dissolve some of the sugars that make up the plant. This may cause the cabbage to be weaker and break more easily.
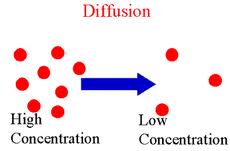
Second, we had to review 8 concepts pertaining to diffusion and osmosis. We learned that diffusion is the tendency of molecules to move from areas of higher concentration, to areas of lower concentration.
We then learned about osmosis, which is basically diffusion involving water. In osmosis however, water has to move through a selectively permeable membrane, which allows some types of molecules, and restricts others.
 |
| Water moves from high concentration to low concentration. |
When a cell is involved, the movement of water is influenced by the solute concentration of the solution. If there is a higher concentration of solute in the cell than in the solution, then the water will move in, if there is smaller concentration of solute in the cell than in the solution, then the water will move out. This is the basis on which we tested our experiment.
For our in-class experimental lab, we created six cells using dialysis tubing, each about 7ml. Then we dried the cells, removing excess water weight, and massed them. Then we put each cell into a 50ml cup of water, covering the cell completely. We let them sit in the cups for 30 minutes, and then re-massed the cells and collected our data. With the exception of two cells, our data showed that the cells gained mass. This means that water moved into the cells, where the water concentration was less.
 |
| A picture of the cells that we used. |
We then re-did this experiment, but put Gatorade inside of all six cells, and and covered each of these cells with 50 ml of the six different colored solutions. We weighed the dried cells before putting them in the solutions, then came back at night to weight the cells after a day of sitting in the cups. Our data showed that the % mass change for each of the cells was negative, and with the exception of one cell, the % mass change of the cells went in the same order as molarities of the solutions in the cells, with % mass changes=low molarity, and high % mass change=high molarity. This shows that the solute in the cells diffused to areas of lower concentration, which in this case was the water. With less solute remaining in the cell, the mass of the cell decreased, and that explains why the cell with the most solute lost the most mass. Since the blue cell had the least % change in mass, it can be reasoned that it had the closest molarity to the gatorade.
We also learned terms such as the following:
Hypotonic- lower solute concentration/higher water concentration
Hypertonic- higher solute concentration/lower water concentration
Water moves from Hypotonic --> Hypertonic
Isotonic- Equal in concentrations
Water Potential- Tendency of water to leave one place in favor of another.
Water moves from Higher Water Potential --> Lower Water Potential
Plasmolysis- process in plant cells where the cytoplasm pulls away from the cell wall due to loss of water through osmosis.
Cytolysis- Occurs in a hypotonic solution. The net flow of water goes into the cell
We also learned terms such as the following:
Hypotonic- lower solute concentration/higher water concentration
Hypertonic- higher solute concentration/lower water concentration
Water moves from Hypotonic --> Hypertonic
Isotonic- Equal in concentrations
Water Potential- Tendency of water to leave one place in favor of another.
Water moves from Higher Water Potential --> Lower Water Potential
Plasmolysis- process in plant cells where the cytoplasm pulls away from the cell wall due to loss of water through osmosis.
Cytolysis- Occurs in a hypotonic solution. The net flow of water goes into the cell


No comments:
Post a Comment